Intensive release of heavy policies, what is the prospect of the API market?
01 Policy Escort
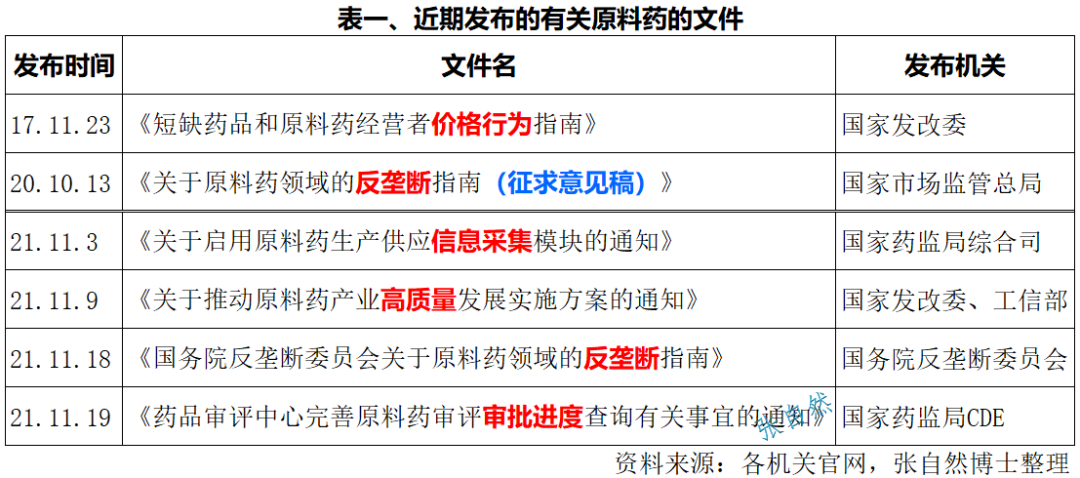
In the past half a month (November 3 to 19), the state has issued four documents in succession specifically for APIs, which is really rare, which shows that the state attaches great importance to the API industry. (Table I)
On the 9th of this month, the National Development and Reform Commission and the Ministry of Industry and Information Technology jointly issued the "Implementation Plan on Promoting the High-quality Development of the API Industry", which clearly stated that by accelerating technological innovation and upgrading, promoting green and low-carbon development, promoting industrial layout adjustment, and promoting international development to a higher level In 2025, it will develop a batch of high value-added and high-growth varieties, break through a batch of green and low-carbon technology and equipment, cultivate a batch of leading enterprises with international competitiveness, and create a batch of industrial clusters and industrial clusters with global influence.
On the 18th of this month, the day the State Anti-Monopoly Bureau was officially listed, the Anti-Monopoly Committee of the State Council issued the "Anti-monopoly Guidelines on the Field of APIs", which is a response to the "Anti-Monopoly Guidelines on the Field of APIs" issued in October 2020. The implementation of the Monopoly Guidelines (Draft for Comments) is also the second time that the country has issued an anti-monopoly enforcement document in the field of raw materials after the National Development and Reform Commission issued the Guidelines for Price Behavior of Operators of Shortaged Drugs and APIs in 2017.
In fact, as early as on the 3rd of this month, the General Department of the State Food and Drug Administration also issued a special document for APIs, namely, the "Notice on Activating the Information Collection Module for API Production and Supply", which aims to strengthen the information infrastructure of APIs. Before (November 19), CDE of the State Food and Drug Administration issued the "Notice of the Center for Drug Evaluation to Improve the Review and Approval Progress of APIs" to improve the transparency of drug review and approval.
The successive issuance of the four consecutive documents has laid a foundation for ensuring the supply and price of drugs in short supply, improving the transparency of the industry, and carrying out anti-monopoly work, and policy guarantees for the healthy and high-quality development of the API industry.
02 Procurement promotion with quantity
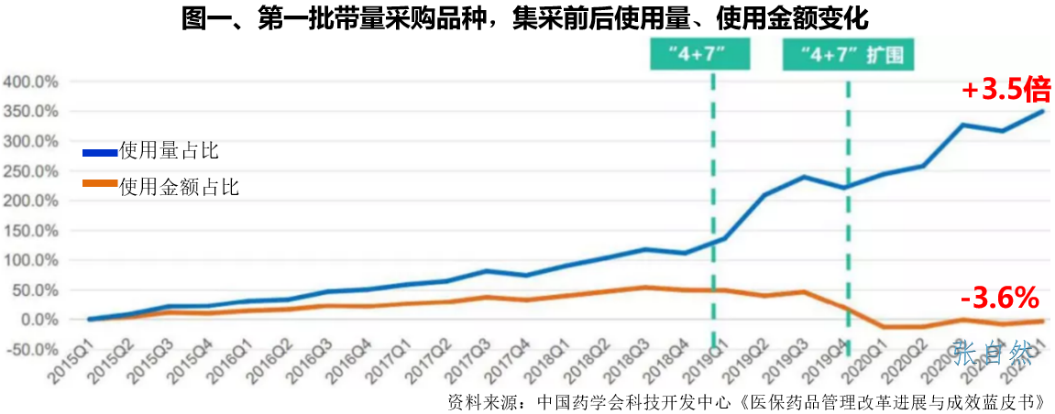
The "Blue Book on the Progress and Effectiveness of Medical Insurance Drug Management Reform" edited by the Science and Technology Development Center of the Chinese Pharmaceutical Association shows that the system design of volume-based procurement has achieved the effect of volume increase and price reduction. Compared with the first quarter of 2021, the first batch of national The consumption of medicines increased by 3.5 times, and the amount of medicines decreased by 3.6%.
The expansion of the use of pharmaceutical preparations has directly driven the increase in the consumption of raw materials. At present, 218 varieties of products have been purchased at the national level.
The "Implementation Opinions of the State Council Leading Group on Deepening the Reform of the Medical and Health System on Deepening the Experience of Sanming City, Fujian Province and Deepening the Reform of the Medical and Health System" issued on October 15 requires:
"Gradually expand the scope of procurement, and strive to purchase more than 300 generic names of drugs by the end of 2022. At the end of the "14th Five-Year Plan" period, the number of generic drug names for centralized procurement of drugs by national and provincial organizations in each province should exceed 500." With the expansion of the varieties purchased with volume, more varieties will achieve a substantial increase in the procurement volume through centralized procurement, and the capacity of APIs will also be expanded accordingly.
03 Export Pull
My country's APIs have always played an important role in the world. Since the outbreak of the new covid-19 epidemic, the supply chains of APIs in many countries have been interrupted. China's epidemic has been fully controlled and production has been fully resumed. The status of my country's APIs in the world has become more prominent.
1. Export
In recent years, my country's raw materials as a whole have shown a continuous growth trend, which has increased from US$23.6 billion in 2013 to US$35.7 billion in 2020.
With the exception of 2015 and 2016, which saw a decline of 1-2%, all other years achieved positive growth.
According to preliminary statistics from the China Chamber of Commerce for Import and Export of Medicines and Health Products, in the first half of 2021, the export value of my country's APIs was US$19.5 billion, a year-on-year increase of 14%, but mainly driven by the 18% year-on-year increase in the average export price, the export volume decreased by 4% year-on-year.
Since the second half of 2020, the unilateral rise in global bulk raw material prices and cross-border logistics costs is the main reason for the sharp rise in the average export price of APIs. In the next few years, my country's API exports will also face the test of excess capacity.

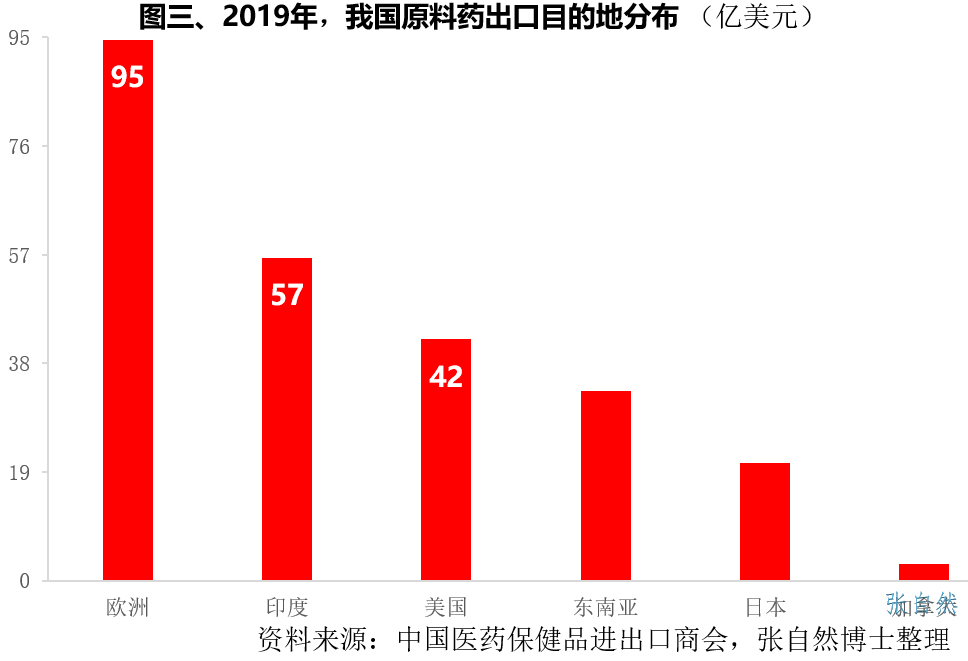
2. Export destination
Europe is the primary destination for my country’s API exports. In 2019, my country’s raw material exports to Europe amounted to US$9.46 billion, followed by India and the United States, which exported US$5.65 billion and US$4.22 billion of APIs respectively in 2019.
04 Patent expired market potential
The price of generic drugs is much cheaper than that of patented drugs, so generic drugs can greatly improve the accessibility of patients, and the usage of generic drugs is significantly higher than that of patented drugs, and generic drugs must wait for the patent of patented drugs to expire. Therefore, the number of expired patents will directly affect the listing process of generic drugs.
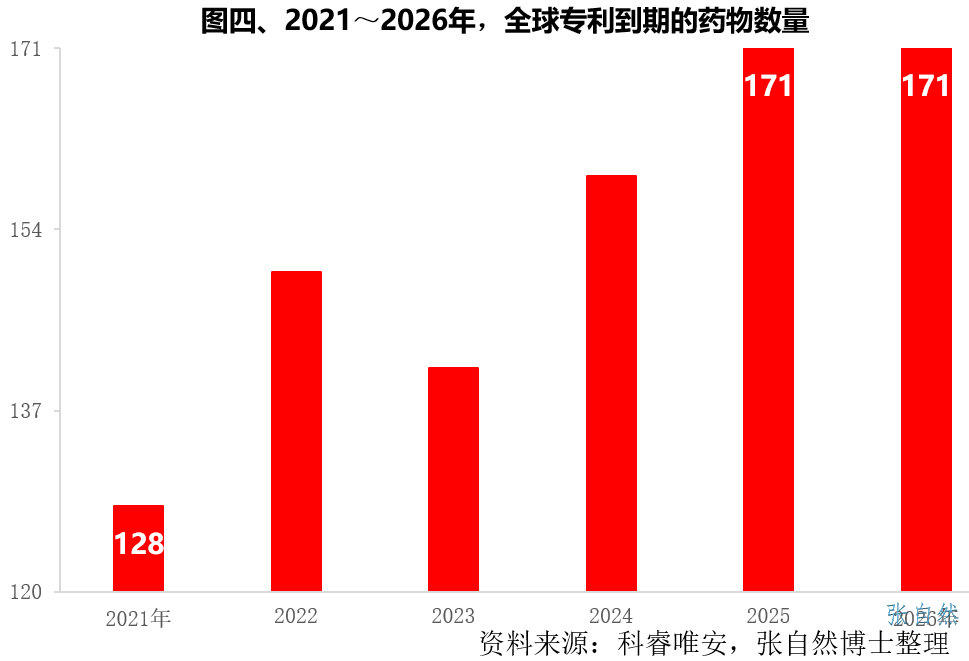
In the next few years, there will be a wave of patent expiration. According to Clarivate Analytics, there will be 128 patent expirations of patented drugs in the world this year (2021), which will increase year by year in the future. 171 patents will expire, so a large number of generic drugs will be launched in the next few years, and the demand for corresponding APIs will also increase.
According to Evaluate Pharma's estimates, from 2021 to 2024, patent drugs with a total sales of 145 billion US dollars will expire, corresponding to about 14.5 billion to 29 billion US dollars of generic drug replacement space.
05 Capital Driven
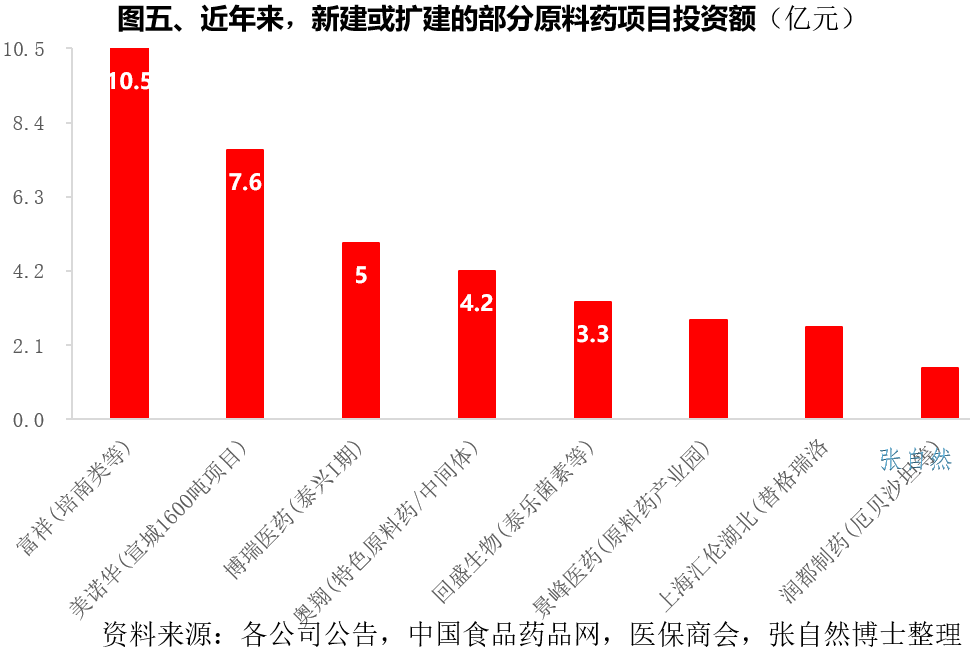
In recent years, the number of new or expanded API projects has been increasing.
On May 8, 2020, the foundation stone laying ceremony of Borui Pharmaceutical (Taixing) biological raw materials and oral preparations project with an investment of 500 million yuan was held in Taizhou. The new project is expected to increase annual sales revenue of 4.7 billion yuan.
On July 20, 2020, Aoxiang Pharmaceutical announced that the total amount of funds raised by the proposed non-public offering of stocks will not exceed 420 million yuan (including this amount), which will be used for the production base of characteristic raw materials and key pharmaceutical intermediates after deducting the issuance costs. construction project.
On August 1, 2020, Fuxiang Pharmaceutical announced that the proposed investment amount is 1.05382 billion yuan to build a high-efficiency penem antibiotic construction project of Jingdezhen Fuxiang Life Technology Co., Ltd. After the project is completed, it will form an annual output of 600 tons of 4AA and 200 tons of meropenem production capacity.
06 Assisted by continuous reaction technology
Continuous production technology refers to transforming the traditional batch production process into an automated continuous process. According to the data of Roots Analysis, through the application of continuous reaction, the comprehensive equipment efficiency of the reactor batch reaction can be increased from the traditional 30% to 75% or more, it can also save 70% of the equipment area and manpower, and reduce the production cost and quality time by about half.
According to Frost & Sullivan, Asymchem is one of the first companies in the world to apply continuous production technology to pharmaceutical production.
With the support of policies and the continuous improvement of supervision, the continuous expansion of the scope of volume procurement, the advent of patent expiration, and the promotion of new technologies such as continuous production technology, my country's API industry will enter a stage of high-quality and rapid development, transformation and upgrading acceleration and industry concentration will also continue to increase.
Statement: All reprinted articles on this website are for the purpose of transmitting more information, and the source has been indicated. The reprinted content does not represent the position of this site. If the copyright owner of the reproduced article does not wish to be reproduced, please contact us and we will delete it immediately.